Shunpu Zhang
Enhancing Retinal Vascular Structure Segmentation in Images With a Novel Design Two-Path Interactive Fusion Module Model
Mar 03, 2024Abstract:Precision in identifying and differentiating micro and macro blood vessels in the retina is crucial for the diagnosis of retinal diseases, although it poses a significant challenge. Current autoencoding-based segmentation approaches encounter limitations as they are constrained by the encoder and undergo a reduction in resolution during the encoding stage. The inability to recover lost information in the decoding phase further impedes these approaches. Consequently, their capacity to extract the retinal microvascular structure is restricted. To address this issue, we introduce Swin-Res-Net, a specialized module designed to enhance the precision of retinal vessel segmentation. Swin-Res-Net utilizes the Swin transformer which uses shifted windows with displacement for partitioning, to reduce network complexity and accelerate model convergence. Additionally, the model incorporates interactive fusion with a functional module in the Res2Net architecture. The Res2Net leverages multi-scale techniques to enlarge the receptive field of the convolutional kernel, enabling the extraction of additional semantic information from the image. This combination creates a new module that enhances the localization and separation of micro vessels in the retina. To improve the efficiency of processing vascular information, we've added a module to eliminate redundant information between the encoding and decoding steps. Our proposed architecture produces outstanding results, either meeting or surpassing those of other published models. The AUC reflects significant enhancements, achieving values of 0.9956, 0.9931, and 0.9946 in pixel-wise segmentation of retinal vessels across three widely utilized datasets: CHASE-DB1, DRIVE, and STARE, respectively. Moreover, Swin-Res-Net outperforms alternative architectures, demonstrating superior performance in both IOU and F1 measure metrics.
Deep Epidemiological Modeling by Black-box Knowledge Distillation: An Accurate Deep Learning Model for COVID-19
Jan 20, 2021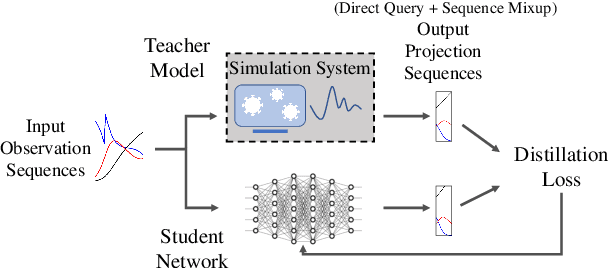
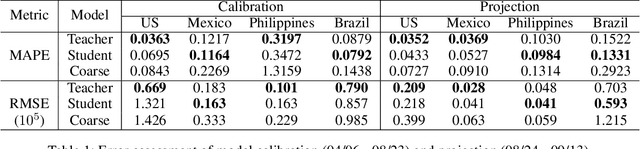
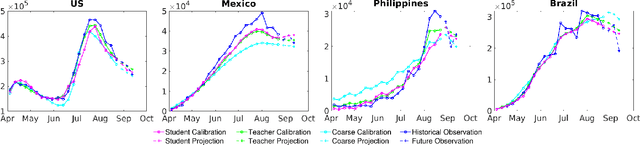
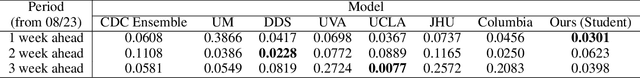
Abstract:An accurate and efficient forecasting system is imperative to the prevention of emerging infectious diseases such as COVID-19 in public health. This system requires accurate transient modeling, lower computation cost, and fewer observation data. To tackle these three challenges, we propose a novel deep learning approach using black-box knowledge distillation for both accurate and efficient transmission dynamics prediction in a practical manner. First, we leverage mixture models to develop an accurate, comprehensive, yet impractical simulation system. Next, we use simulated observation sequences to query the simulation system to retrieve simulated projection sequences as knowledge. Then, with the obtained query data, sequence mixup is proposed to improve query efficiency, increase knowledge diversity, and boost distillation model accuracy. Finally, we train a student deep neural network with the retrieved and mixed observation-projection sequences for practical use. The case study on COVID-19 justifies that our approach accurately projects infections with much lower computation cost when observation data are limited.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge